On May 15, 2024, Sungening Biotech's first innovative drug SG1001 successfully obtained implied clinical trial approval from the National Medical Products Administration (acceptance number: CXHL2400203) and is about to commence clinical trials. This marks that Sungening Biotechnology has successfully become an innovative pharmaceutical company in the clinical stage. SG1001 has been approved for invasive fungal disease (IFD). IFD refers to the pathological and physiological processes in which pathogenic fungi invade human tissues and blood, grow and reproduce, causing tissue damage, organ dysfunction, and inflammatory reactions, posing a serious threat to the lives and health of low-income populations.
At present, the development of innovative drugs in the field of antifungal infections in China is slow, seriously lagging behind the needs of China's economic and social development. The approval of SG1001 for clinical trials will be an important step forward in our efforts in the field of antifungal infections. At the same time, Sungening Biotechnology is very grateful for the strong support from relevant departments and cooperative units, especially Haibowei, Medixi, Kailaiying, Xinlitai, and Taige.
IFD causes over 1.5 million deaths annually, particularly among elderly individuals with immunodeficiency, organ transplantation, severe pneumonia, and underlying diseases. The aging population in the world is becoming increasingly severe, and the demand for drugs to treat IFD in both international and domestic markets is growing. Due to the difficulty in finding drug targets compatible with the human body, the available antifungal agents on the market are extremely limited and have significant toxic side effects. In addition, the emergence of drug-resistant strains urgently requires the development of new antifungal drugs to meet current clinical needs.
In 2022, the World Health Organization (WHO) released the "Priority List of Pathogenic Fungi", which listed common clinical pathogens such as Aspergillus fumigatus, Cryptococcus neoformans, Candida albicans, and Candida auricula as key priority pathogens, attracting international attention. Especially Aspergillus fumigatus, widely distributed in the environment, mainly infects the lungs and is prone to developing drug resistance. The incidence and mortality rates of pulmonary aspergillosis are relatively high worldwide, requiring long-term antifungal treatment.
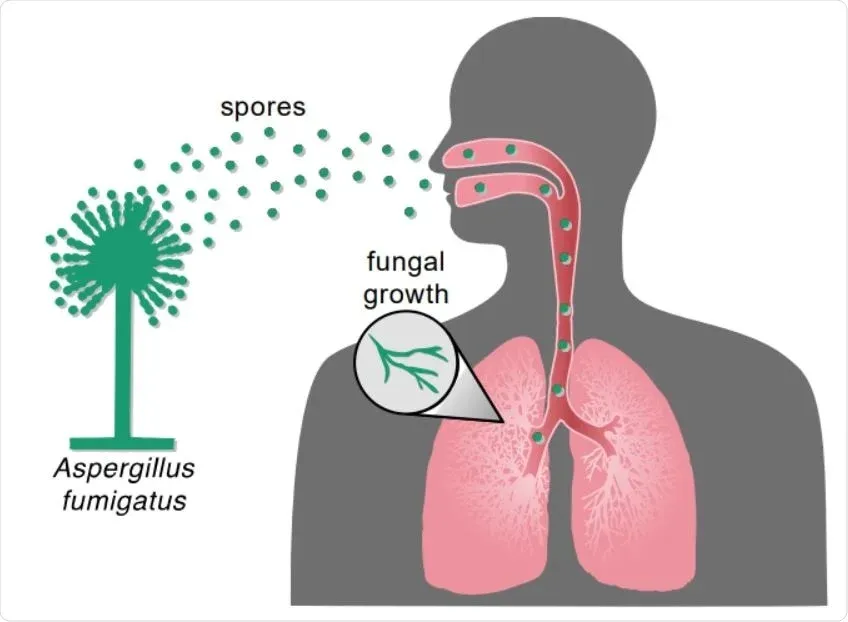
SG1001 is a selective inhibitor of fungal dihydroorotate dehydrogenase (DHODH), which exhibits significant antibacterial activity against Aspergillus (including Aspergillus fumigatus), Pseudomonas aeruginosa, Penicillium, Trichoderma, and Nepenthes marnefei. Fungal DHODH is a key enzyme in the biosynthesis of pyrimidine in its cells, which inhibits the activity of DHODH in fungal cells to block the pyrimidine biosynthesis pathway and exert antifungal effects. This mechanism of action is different from common triazole, polyene, and echinocandin antifungal agents.
SG1001 is the world's second DHODH inhibitor, with preclinical pharmacokinetic data superior to F2G's Olorofim (F901318) from the UK, and possessing some of the characteristics of Best in Class. Olorofim is currently undergoing Phase 3 clinical trials, with Phase 2 trials being successful for patients diagnosed with invasive fungal infections or suspected pulmonary aspergillosis with limited treatment options. The study included 203 patients with limited alternative therapies, such as failed available therapies, resistance to all licensed drugs, intolerance to existing therapies, drug interactions, and inability to achieve therapeutic levels. Olorofim has shown potential in addressing the high unmet demand for antifungal therapy, particularly against drug-resistant fungi. With the increasing prevalence of individuals at risk of infection and with weakened immune function, new therapies like Olorofim are expected to have a significant impact on the treatment of patients.




